The ROBODOC Surgical System
The complete ROBODOC® Surgical System utilizes the tandem technologies of CUREXO Technology Corporation’s ORTHODOC® and ROBODOC® Surgical Assistant. Enabling pre-surgical 3D planning, ORTHODOC® provides exceptional accuracy in component selection, placement, surface preparation and soft tissue management. Meanwhile, the ROBODOC® Surgical Assistant implements the pre-surgical plan with unparalleled precision.
The ORTHODOC® Preoperative Planning Workstation provides the surgeon with 3D information and easy point-and-click control. The pre-surgical planning begins as ORTHODOC converts a CT scan of the individual patient’s joint into a 3-dimensional virtual bone image which the surgeon can manipulate to view bone and joint characteristics. In this way ORTHODOC® enables the surgeon to perform a simulated surgery allowing for exploration of several surgical plan options without risk to the patient or expending valuable surgical time.

A prosthetic image is selected from the ORTHODOC’s extensive digital library of options from major manufacturers. The surgeon is then able to manipulate the three-dimensional model of the prosthesis against the CT bone image, allowing for optimal prosthetic selection and alignment. Through ORTHODOC®, this virtual surgery creates a precise preoperative plan that is meticulously customized for each patient.
The preoperative plan created on ORTHODOC® is then electronically transferred to the ROBODOC® Surgical Assistant. This advanced surgical robot can execute the preoperative plan with a degree of precision and reliability that is unparalleled. Using controlled, gentle pressure the ROBODOC® mills the bone with sub-millimeter accuracy exactly as specified by the plan. The action of the high-speed drill equipped robotic arm has also proven to be more precise than manual preparation techniques. ROBODOC’s specialized drill bits and other hardware have been developed for accurately preparing the bone to achieve optimal fit of the prosthetic implant. The robot mills cavities for hip implants, removes bone cement for revision surgeries, and planes the femoral and tibia surfaces for knee implants.
Based on extensive clinical studies ORTHODOC® and the ROBODOC® Surgical Assistant work together seamlessly to ensure the ROBODOC® Surgical System provides better overall precision in total joint replacement procedures. Benefits of the ROBODOC® System over traditional methods include improved cavity fit, fill and alignment for prosthetic implants1,2,4 as well as a reduction of leg length discrepancy2, intra-operative fractures1,3 and pulmonary emboli5.
FAQs
Overview
The ROBODOC® Surgical System consists of two components: ORTHODOC®, a three dimensional (3-D) workstation for preoperative surgical planning, and the ROBODOC® Surgical Assistant, a computer-controlled surgical robot utilized for precise bone cavity and joint surface preparation for total hip arthroplasty (THA) and total knee arthroplasty (TKA) surgeries. Preoperative planning with ORTHODOC® begins as a CT scan of the patient’s affected joint is converted into a 3-D virtual bone image that can be manipulated and viewed in any plane. The surgeon selects and positions a 3-D image of the prosthesis within the reconstructed CT bone image that best fits the patient. Using this surgeon-formulated plan, the ROBODOC® Surgical Assistant then precisely mills the bone / joint to accept the implant. The ROBODOC® Surgical System is the only active robotic system cleared by the Food and Drug Administration (FDA) for use in orthopedic surgery in the U.S. The system has been used in over 28,000 THA and TKA* surgical procedures worldwide. [*TKA not for sale in the U.S.]. ROBODOC® and ORTHODOC® are registered trademarks of Curexo Technology Corporation (CTC).
Q: What is the difference between the ROBODOC® System and surgical navigation?
A: Various computer navigation systems now widely employed in orthopedic surgery assist the surgeon in the execution of the procedure by providing intraoperative 3-D images of the patient’s anatomy. However, some navigation systems are “image free,” requiring the surgeon to locate various bone landmarks to “register” the navigation system to a generic anatomy. This introduces possible errors due to the difficulty of accurately locating these landmarks. Additionally, even if the navigation data is very accurate, the surgeon must then execute the plan using manual instruments that cannot match the precise bone / joint preparation afforded by ROBODOC®. In contrast, ROBODOC®’s DigiMatch™ software (introduced in 2000 to obviate a previous pin-based registration) allows for CT surface-based registration of the patient’s distinct anatomy. Rather than identify specific landmarks, the surgeon selects points in specified regions of the bony surface. Using the preoperative plan implemented in ORTHODOC®, ROBODOC® then executes the bone preparation exactly as planned with minimal chance for error. Figure 1 demonstrates the DigiMatch™ registration process.
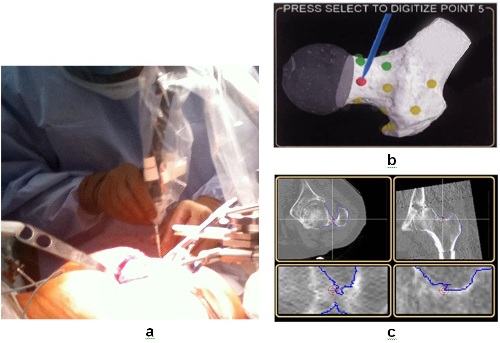
Figure 1. The procedures of DigiMatch™ registration. a) During surgery, the surgeon uses a “digitizer” to locate the patient’s anatomy by selecting points on the femoral surface. b) The monitor displays the general locations of the points to be registered on the proximal femur. DigiMatch™ technology matches these to the bone surface model generated preoperatively by ORTHODOC®. c) The surgeon verifies the accuracy of the registration by touching bone surfaces with the digitizer. If the selected location is within the target on the bone surface as shown on the monitor (red crosshair targets on the blue bone surfaces), the surgeon accepts the registration. (Adapted from Nakamura et al., 2009).
Q: What kind of accuracy can be achieved with bone / joint preparation with the ROBODOC® System?
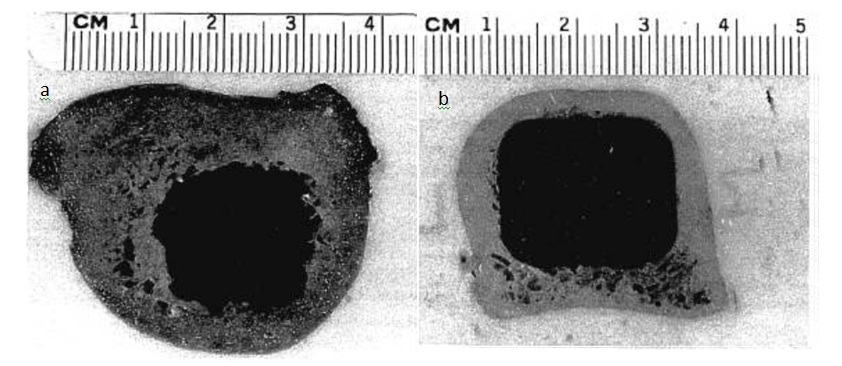
A: ROBODOC® provides for a cutting accuracy of less than 0.4 mm. This provides for excellent bone-to-implant contact. For example, studies with human cadaveric femoral bones demonstrated 96% contact between bone and femoral stem implant when the cavity was milled by ROBODOC® versus only 21% bone-to-implant contact when femoral stems were placed by hand/mallet driven reamers and broaches (Paul et al., 1992). Figure 2 shows the precise intramedullary milling achievable with ROBODOC® compared to manual surgical techniques.
Q: What type of implants can I use with the ROBODOC® System?
A: ROBODOC® offers an open implant platform with a library of the 3-D shapes and sizes of many commonly used hip and knee implants. This implant library is continually updated and ultimately can incorporate any manufacturers implant.
Q: What is the learning curve for the ORTHODOC® pre-surgical planning 3-D workstation?
A: While ROBODOC® is a unique and advanced system, CTC will provide all of the training you and your staff need to become proficient to take advantage of the superior performance and results ROBODOC® offers for THA and TKA procedures.
This training includes visits by interested surgeons to observe ROBODOC® THA procedures performed by Dr. William Bargar (Assistant Clinical Professor of Orthopedic Surgery, UC Davis School of Medicine, Sacramento, CA) and subsequent visits by Dr. Bargar and other CTC Clinical Trainers to assist in ROBODOC® procedures at your facility. Additionally, information on each surgical procedure is recorded on a CD disc for review and analysis by CTC to assess issues relating to surgeon and robot performance. Analysis of this data allows CTC to determine whether additional surgeon or surgical staff training is needed and to evaluate potential problems with the ROBODOC® Surgical Assistant device.
Q: Can ROBODOC® place or assist in the placement of the acetabular cup?
A: The ROBODOC® Surgical Assistant was not designed for placement of the acetabular cup. However, ORTHODOC® presurgical planning assists in proper acetabular cup placement; the degree of femoral anteversion, assessed precisely from the pre-OP CT scan with the ORTHODOC® workstation, can be used to determine the target position of the acetabular component, and thereby assure maximum hip joint ROM without impingement.
Q: How much time does it take to plan a case with ORTHODOC®?
A: Once training is complete and one is familiar with the system, planning a complete THA procedure takes 20-30 minutes. Typically, the preoperative planning for a TKA case takes about 30-45 minutes. Examples of ORTHODOC® preoperative planning for THA and TKA cases are shown in Figures 3 and 4, respectively. [TKA not for sale in the U.S]
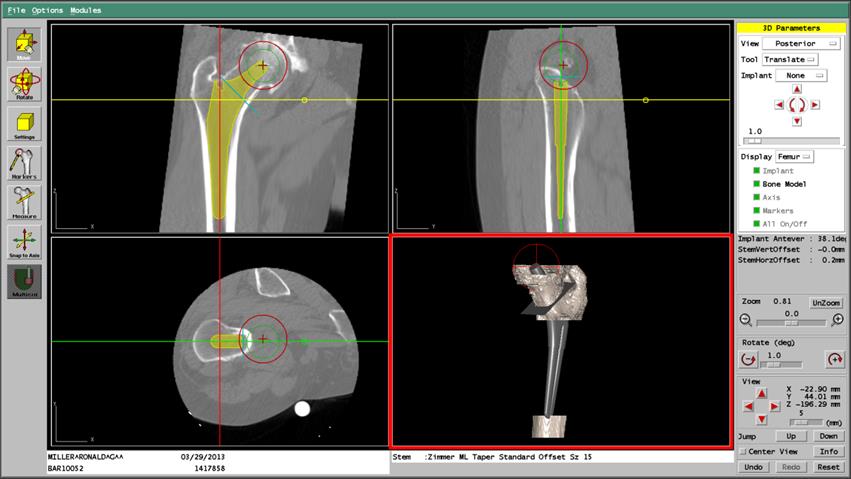
Figure 3. ORTHODOC® Preoperative THA Planning; Femoral Stem Placement. An ORTHODOC®workstation screen shot showing AP, lateral and sagittal views of an implant positioned in reconstructed CT images of the femur and the implant within in a 3-D Surface Model of the bone showing the plane of the femoral neck osteotomy, and the outline of the position of the new femoral head (red circle).
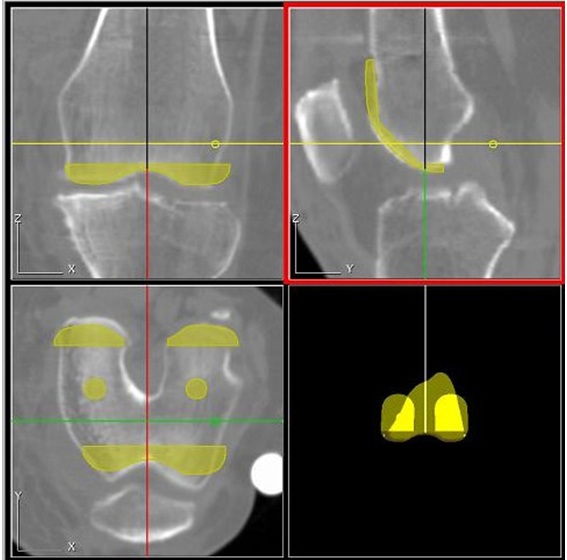
Figure 4. ORTHODOC® preoperative TKA planning. Preoperative AP, lateral and sagittal plane CT views of a positioned femoral implant. [TKA not for sale in the U.S]
Q: How much time does it take to perform a THA or TKA case with ROBODOC® compared to conventional surgery?
A: As with most new procedures, total surgical time decreases with continued operator use and experience. Studies directly comparing conventional and ROBODOC® THA indicate an additional 12-20 minutes were required for a ROBODOC® THA procedure, and that ROBODOC® femoral milling time was approximately 13 minutes (Nishihara et al., 2006, Nakamura et al. 2010). Most of the extra surgical time in ROBODOC® THA procedures was consumed by setup, femur fixation / distal leg stabilization and registration.
ROBODOC® TKA took an average of 25 minutes longer than conventional TKA (Song et al., 2012). [TKA not for sale in the U.S.]
Q: Where can one find clinical data comparing THA and TKA surgery with ROBODOC® to manual procedures?
A: Extensive clinical data on THA and TKA procedures performed by ROBODOC® has been published and can be found below. [TKA not for sale in the U.S.]
ROBODOC THA Publications
- Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Relat Res. 1998 Sep;(354):82-91.
- Börner M, Bauer A, Lahmer A. [Computer-guided robot-assisted hip endoprosthesis]. Orthopade. 1997 Mar;26(3):251-7. German.
- Hagio K, Sugano N, Takashina M, et al. Effectiveness of the ROBODOC system in preventing intraoperative pulmonary embolism. Acta Orthop Scand. 2003 Jun;74(3):264-9.
- Hananouchi T, Sugano N, Nishii T, et al. Effect of robotic milling on periprosthetic bone remodeling. J Orthop Res. 2007 Aug;25(8):1062-9.
- Nakamura N, Sugano N, Nishii T, et al. Robot-assisted primary cementless total hip arthroplasty using surface registration techniques: a short-term clinical report. Int J Comput Assist Radiol Surg. 2009 Mar;4(2):157-62. Epub 2009 Feb 13.
- Nakamura N, Sugano N, Nishii T, et al. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop Relat Res. 2010 Apr;468(4):1072-81. Epub 2009 Nov 5
- Nishihara S, Sugano N, Nishii T, et al. Comparison between hand rasping and robotic milling for stem implantation in cementless total hip arthroplasty. J Arthroplasty. 2006 Oct;21(7):957-66.
- Paul HA, Bargar WL, Mittlestadt B, et al. Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res. 1992 Dec;(285):57-66.
- Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007 Dec;3(4):301-6.
- Börner M, Wiesel U, Ditzen W. Clinical experiences with Robodoc and the Duracon total knee. In: Stiehl JB et al., editors. Navigation and Robotics in Total Joint and Spine Surgery, Springer-Verlag:362-366, 2004.
- Park SE, Lee CT. Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty. 2007 Oct;22(7):1054-9.
- Song EK, Seon JK, Park SJ, et al. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2011 Jul;19(7):1069-76. Epub 2011 Feb 11.
- Song EK, Seon JK, Yim JH, et al. Robotic-assisted TKA Reduces Postoperative Alignment Outliers and Improves Gap Balance Compared to Conventional TKA. Clin Orthop Relat Res. Epub 2012 Jun 6.
- Sugano N, Kakimoto A, Kakamura N. Robodoc Total Knee Arthroplasty, Department of Orthopaedic Surgery, Osaka University Graduate School of Medicine. Presented at: 2nd Annual Conference of the Indian Society of Hip and Knee Surgeons; 2008, April 26; Chennai, India.
ROBODOC TKA Publications. [TKA not for sale in the U.S.]
Q: Compared to traditional manual surgery, what improved intra- / peri-operative and short -term measures, and long-term functional outcomes have been documented with ROBODOC® surgery?
A:For THA procedures done with ROBODOC®, there were no intra-operative femoral fractures caused by implant insertion (Börner et al., 1997; Bargar et al., 1998; Nishihara et al., 2006, Nakamura et al., 2010) and a decreased incidence of blood clots / pulmonary embolism (Hagio et al., 2003). Improved short term measures included improved implant fit, fill and alignment (Bargar et al., 1998; Nishihara et al., 2006, Nakamura et al., 2010). Regarding long-term outcomes, the more precise fit, fill and alignment of the femoral stem provided for more bone-implant spot welds (Hananouchi et al., 2007), less stress shielding (Hananouchi et al., 2007; Nakamura et al., 2010), decreased proximal periprosthetic bone loss (Nakamura et al., 2010), and a decreased incidence of leg length discrepancies (Nakamura et al., 2010). Patients undergoing ROBODOC® THA procedures experienced similar average length of hospital stay, and similar SF-36 survey and Harris Hip osteoarthritis index scores compared to conventional manual THA (Bargar et al. 1998).
For TKA procedures, no mechanical axis alignment outliers were observed with ROBODOC® compared to 24% for conventional surgery (Song et al., 2012). Additional improved outcomes with ROBODOC® included better flexion-extension gap balance. No differences were detected between ROBODOC® and conventional TKA procedures in postoperative ROM or in WOMAC and Hospital for Special Surgery (HSS) scores (Song et al., 2012). [TKA not for sale in the U.S.]
A list of published studies comparing various clinical outcomes between manual and ROBODOC® THA and TKA procedures can be accessed with the link in the previous question.
Q: What are the plans for expansion and continued development of the ROBODOC® Company in the future?
A: CTC will continue to support and expand our current markets, and we are building our company structure to support expansion in selected global markets. Long-term, ROBODOC® systems will be installed worldwide including markets in Asia, Europe, Australia and South America. Ultimately we will have a direct sales force presence in each of the larger markets and distributors for the smaller ones.
Q: What is the status of the ROBODOC® System’s FDA clearance for other applications in the U.S.?
A: CTC received clearance for THA surgery from the U.S. FDA in 2008 and will be seeking clearance for TKA procedures in the U.S.
Q: What are some of the future applications of the ROBODOC® System?
A: Because ROBODOC® is multi-platform system, the existing hardware can easily incorporate new application modules. Currently new procedures are being developed for applications such as hip revision. Future applications may include other joint procedures.
Q: What improvements are being made to make the ORTHODOC® software more user-friendly?
A: We are constantly working to improve the efficiency and usability of the entire ROBODOC® System, and actively seek input from users. Improvements to ORTHODOC® software and to the ROBODOC® Surgical Assistant® are released periodically.
Q: How many registration points are taken during surgery and how long does this take?
A: For THA, 17 surface points are collected along with 2 recovery marker points. This process takes 8-12 minutes. For TKA, 42 total points are taken requiring 12-26 minutes. [TKA not for sale in the U.S.]
Q: What types of fixation methods are currently used?
A: For THA cases, two 5mm Schanz pins are placed in the femoral head to securely fix ROBODOC® to the patient’s femur. A CTC-supplied Distal Leg Fixation Device is used to prevent movement of the distal leg. For TKA procedures, two 5 mm Schanz pins are used; one in the lateral femur and one in the lateral tibia. In addition to the pins that are secured to ROBODOC®, a De Mayo Knee Positioner™ (Innovative Medical Products, Inc.) prevents distal leg movement. [TKA not for sale in the U.S.]
Q: What is the purpose of the recovery markers in THA and TKA procedures and can they be eliminated?
A: The purpose of the markers is to provide a reference plane that will allow for relocation of the bone(s) in 3D space and thereby allow the surgery to continue with minimal lost time. The markers are needed because the original surface areas required for registration will have been removed by milling during the robotic surgical process.
Q: What is the Bone Motion Monitor (BMM)?
A: The BMM is a safety feature that detects unintended bone motion after fixation and immediately stops the cutting process if movement reaches a threshold of >2mm for >1 second. For THA procedures one BMM is positioned on the proximal femur, whereas two BMMs (one on the femur and one on the tibia) are used for TKA. If bone motion is sensed, the new location of the bone(s) is determined by locating recovery markers; for THA this takes approximately 3-5 minutes, and for TKA approximately 3-5 minutes for each bone, and the surgery can proceed as planned.
Q: What controls does the surgeon have during robotic milling and how are these displayed?
A: The surgeon has a hand-held pendant with PAUSE and STOP buttons. The PAUSE button stops the motion of the arm and the cutter, allowing the surgeon to reassess and resume the procedure. The STOP button immediately removes power from ROBODOC®’s electromechanical arm. The position and progress of the cutter are displayed in real time on a monitor that shows the portion of the bone that has been removed and that which remains to be excised.
Q: What internal safety controls does ROBODOC® have?
A: Safety and precision in ROBODOC® are achieved by control of the cutter fed rate, speed and contact force. Sensors monitor the forces applied by the cutter to ensure that pre-defined thresholds are not exceeded. ROBODOC® is designed so that no single point of failure can cause uncontrolled arm movement. The system uses several independently operating computers and microprocessors that if not in agreement deactivate power to the robot and the cutter.
In over 900 THA cases monitored in Germany, no awkward performances of the robot and no unpredicted or dangerous movements were observed (Bargar et al., 1998). Additionally, in no case did a surgeon find it necessary to do an emergency stop of a procedure.